Introduction
Words elicit emotions—a concept often exploited by politicians and marketing professionals. The higher the stakes, the higher the emotional impact of words. In medical device regulatory affairs the stakes can be substantial. It is thus not surprising that certain words can provoke strong reactions in this field. For instance, the term clinical investigation often causes anxiety among regulatory affairs managers, as it feels loaded with risk and additional workload. Similarly, the word study—despite lacking an official regulatory definition—is commonly perceived as academic and closely associated with the clinical realm. In contrast, the word survey has a soothing and reassuring effect. Surveys feel familiar, we encounter them every day in the news, in marketing research, in polling. This stands in stark contrast to clinical investigations, with which most people have little familiarity. You hear terms like “clinical study” or “clinical investigation,” but never “clinical survey.” Therefore, surveys do not carry a clinical connotation. Moreover, surveys are mentioned in relevant standards and guidance as legitimate data collection methodologies. “High-quality surveys” are even ranked as level 4 clinical evidence in MDCG 2020-6. As a result, surveys offer the reassuring impression of a systematic and relevant approach to data collection while appearing to sidestep clinical investigations altogether. Saying that surveys cannot be clinical investigations is as misguided as saying that all surveys are for election polling. In reality, the definition of surveys specifies a methodology for data collection. Instead, the definition of clinical investigation specifies an objective—demonstrating performance and safety. This means that the methodology of surveys can also be applied to the purposes of a clinical investigation, Figure 1.
Figure 1: Surveys can be clinical investigations. The term “survey” refers to a methodology used for gathering data, which can be effectively utilized for the purposes of a clinical investigation.
This situation is reminiscent of the challenges we encountered in the classification of medical device software1. The industry expects rules based on clear-cut semantics—if it is a “survey,” then it is not a “clinical investigation.” However, in practice, establishing whether a survey falls under the MDR definition of clinical investigation depends on the medical context and the specific characteristics of the survey. Since it is impossible to explore all possible contexts, here we analyze the impact of surveys characteristics on their regulatory classifications. Indeed, the MDCG guidelines and ISO standards highlight different characteristics of surveys. These are target population, content, and direction. First, however, let’s establish a clear definition of what a survey is.What is a survey
The MDR does not provide a definition for the term survey. In fact, it does not mention the word survey at all. It is the MDCG that proposes using surveys for clinical or post-market clinical follow-up in MDCG 2020-6, MDCG 2020-7, and MDCG 2020-8. However, these guidelines do not define the term either. Similarly, ISO/TR 20416:2020, which recommends surveys for post-market surveillance, does not offer a definition. For a definition, we thus refer to the ISO norms on marketing research. Specifically, ISO 20252:2019 defines a survey as “data collection from a sample of a target population to which inferences can be made.” In essence, to conduct a survey one must:- Select a target population: Identify the group about which inferences are to be made.
- Choose a representative sample: Select a subset of the population that accurately reflects the target population, allowing for the generalization of results.
- Gather data directly from the sample: Collect data through direct interaction, such as questionnaires or interviews. This directness—the process of asking questions directly to the individuals involved—is the defining characteristic of a survey
Target population
The target population refers to the specific group of individuals from whom data is collected. Defining the target population is a fundamental aspect of designing and conducting surveys, as it directly influences the relevance, applicability, and regulatory classification of the collected data. A common misconception is that if a survey targets users, then it cannot be a clinical investigation. This is particularly relevant for usability studies, which are often conducted in the form of surveys from users. MDCG 2021-6 points out that such usability tests ”may have a medical purpose which is evaluated in the usability test and they would thus be considered as investigational devices, whereby the test could be a clinical investigation, in particular when the usability test involves exposing users to risk related to the device or where poor usability may lead to patient or user safety risks.” User surveys—in particular prospective surveys (see Section 5)—can fall under the definition of a clinical investigation if they assess a medical device’s safety or performance and involve potential risks to users. Another common misconception concerns the use of surveys from healthcare professionals (MDCG 2020-7). Surveying healthcare professionals does not mean asking them about patient records. Analyzing data from previously collected patient records fall under retrospective studies, discussed in Section 5. Instead, as discussed in Section 4, surveys from healthcare professionals can be used to identifying signals that a device may not be fulfilling its intended purpose in real-world settings. Finally, let’s consider patients. Before we proceed, it is crucial to clarify the distinction between “user” and “patient”. User and patients are distinct roles, not necessarily distinct individuals. The same physical person can cover both role. The user is any healthcare professional or lay person who uses a device (MDR, Article 2(37)). The patient is any individual under the care of a healthcare provider who may benefit from the action of a medical device (IMDRF WG/N47). In theory, patient survey are not necessarily clinical investigations. In our experience, however, prospective survey targeting patients (see also Section 5) are likely to be clinical investigations. Indeed, manufacturers rarely go to the lengths of contacting patients solely to ask generic preference questions (see Section refsec:content). Such interactions with patients often drift into collecting data directly related to the device’s safety or performance as experienced by the patient, which aligns with the definition of a clinical investigation under the MDR.Content
Survey content plays a major role in determining whether a survey is considered a clinical investigation. Under MDR, a clinical investigation is any systematic investigation— including surveys—that involves humans to assess the performance and safety of a medical device. What is measured, therefore, is key to assessing whether the investigation should be regulated according to the provisions for clinical investigations. In Section 5, we discuss what constitutes human “involvement.” In this section, our focus is on the performance and safety assessment. Every medical device must demonstrate performance, which can be clinical, non-clinical, or related to a medical benefit. Additionally, any device must have a list of expected side effects and residual risks, with their frequency of occurrence. If you conduct a survey with the intention of using the data to support claims about the device’s performance, clinical performance, or medical benefits, or to assess the accuracy of the listed side effects and risks and their probabilities, then you are conducting a clinical investigation, provided that your survey involves human subjects (see Section 5). Survey content that does not fall under the definition of clinical investigation includes usability aspects not related to performance and safety. Beyond usability, surveys can help validate the manufacturer’s understanding of the state of the art or even establish the state of the art in cases where relevant literature is lacking. Surveys are also particular effective tool for post market surveillance. Indeed, there are good reasons why the MDCG guidelines and the standards emphasize the use of surveys to gather preferences and experiences in the post-market phase. First, surveys appear like a natural choice when the device has received market approval. At that point, the device is widely available, and feedback can be collected from users and healthcare professionals without requiring investigational device authorization (see also Section 5). Second, surveys are particularly well-suited for vigilance, for identifying “signals” that a device may not be fulfilling its intended purpose in real-world settings. These signals are not patientspecific data, but general feedback concerning the user experience with the device, for example:- Malfunctions, underperformance (also compared to similar devices), or deterioration in performance;
- Hazards, risks, or hazardous situations experienced by the user or patient;
- mismatch between the rates of expected side effects or residual risks stated by the manufacturer and the healthcare professional’s experience in the praxis;
- unexpected side effects observed by the healthcare professional;
- usability issues;
- insights into the healthcare professional’s preferences in using the device, which may help identify off-label or abnormal use;
- inadequacies of the information material;
- and performance or safety issues in sub-populations.
Direction
An important aspect to consider when evaluating a survey or study is its direction— whether it is retrospective or prospective. This distinction is closely tied to the issue of patient involvement. Retrospective surveys involve collecting data by asking individuals about events or experiences that occurred before the initiation of the survey. In these surveys, data are generated after the survey starts, as participants provide new information based on their recollections of past events. This approach contrasts with retrospective studies, where researchers analyze data that were generated prior to the study—such as hospital records, administrative databases, or previously collected datasets—without interacting with participants to collect new information (see Figure 2). This means that with retrospective surveys—unlike with retrospective studies—researchers can design ad hoc questions tailored to specific research objectives. However, a significant concern in retrospective surveys is the potential for recall bias. This occurs when participants do not accurately remember past events, leading to misreporting or distortion of information. Due to the potential inaccuracies in self-reported data, retrospective surveys generally provide a lower level of evidence compared to studies using prospectively collected data or objective records. However, these surveys can be useful for vigilance activities, as seen in Section 4. The involvement of subjects for data collection in retrospective surveys that aim to assess the performance and safety of a device places these surveys in an intermediate position between retrospective studies and observational/real-world studies, Figure 2. In observational studies, the medical device is used according to normal clinical practice, and the assignment of patients to a particular therapeutic strategy is not predetermined by a trial protocol. Instead, the study’s objective is solely to define the data collection strategy without influencing patient treatment decisions. As explained in MDCG 2020-6, in “retrospective studies the involvement of human subjects and their exposure to the device precedes the study itself as data have already been generated.” Therefore, retrospective studies do not fall under the MDR definition of a clinical investigation because they analyze pre-existing data without new interaction with human subjects.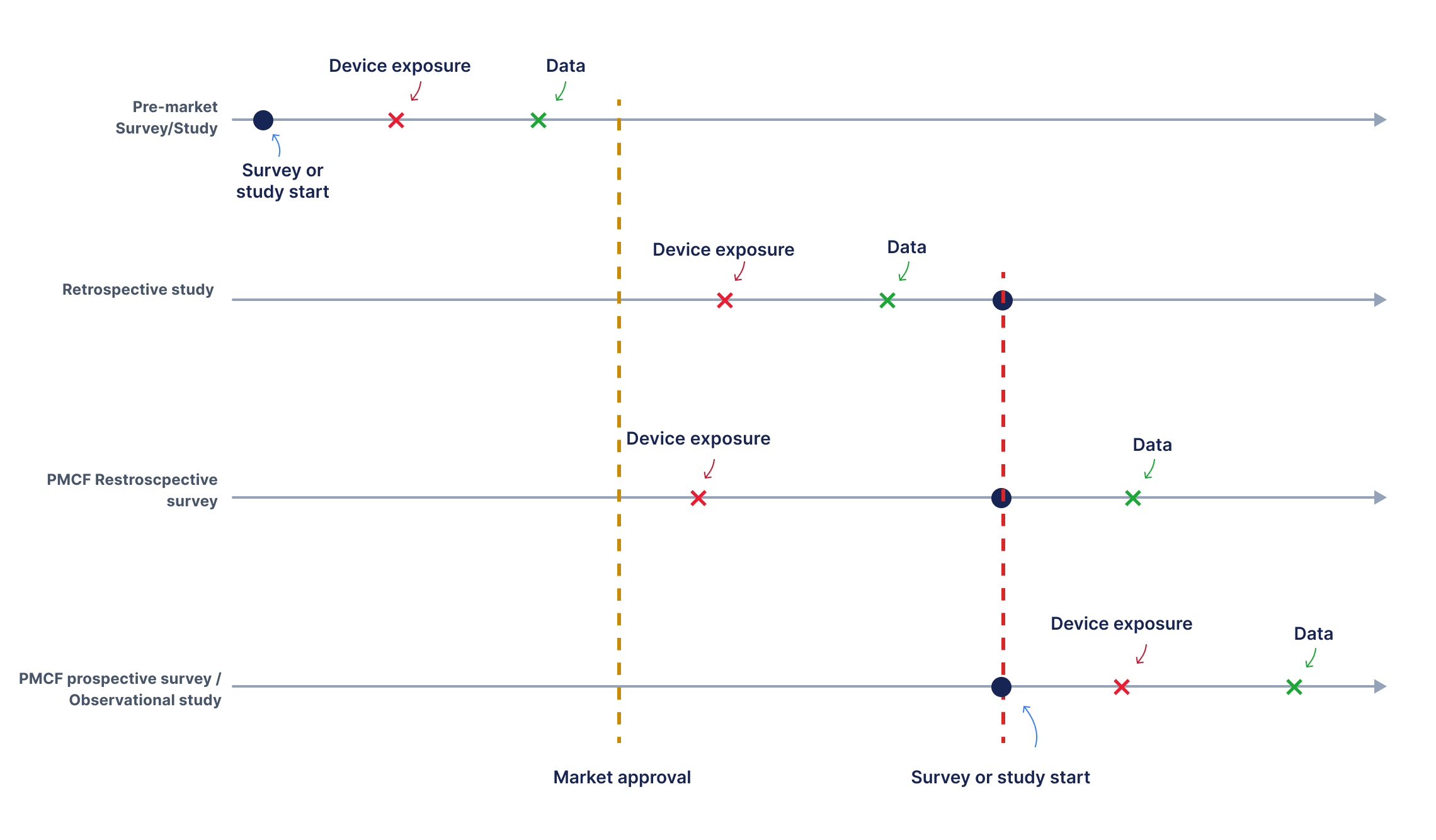
Figure 2: Timing relationships between device exposure, data collection, and the initiation of studies/ surveys across different configurations. In pre-market studies and surveys, data is collected before the device receives market approval, necessitating special authorization to expose patients to the device for study purposes. Once the device is certified, gathering data on its standard use becomes more straightforward from a regulatory standpoint. If both device exposure and data collection occur before the study begins, the study is classified as retrospective and is not considered a clinical investigation according to the MDR, since participant involvement and exposure precede the study. In retrospective surveys, data is collected after the study has commenced, concerning device exposure that occurred prior to the study. In prospective studies/surveys both exposure to the device and data collection follow the study/survey start.
The classification of retrospective surveys is less clear. Unlike retrospective studies, where the exposure of participants to the device precedes the survey without further interaction, retrospective surveys involve direct interaction with human subjects to obtain data. This involvement aligns more closely with the MDR’s definition of a clinical investigation compared to retrospective studies. Therefore, the regulatory classification of retrospective surveys is more nuanced, and these surveys might be considered clinical investigations under the MDR, especially if their content aligns with the purpose of assessing device performance and safety. Prospective surveys are designed to collect data moving forward, rather than relying on past records or patient recollections, Figure 2. Patient involvement in prospective surveys is therefore obvious. When these surveys aim to assess a device’s performance and safety, they function as observational studies based on Patient-Reported Outcomes (PROs). Prospective surveys can also be conducted pre-market (before certification). In the pre-market phase, prospective surveys that assess safety and performance are subject to the provisions of MDR Article 62 and require authorization for the use of the investigational device, just as any other clinical investigation.Conclusion
MDCG 2020-6 places high-quality surveys among level 4 clinical evidence, thus classifying them as potentially suitable even for class III legacy devices and implantable legacy devices that are not well-established technologies. Although the guideline does not explicitly define what constitutes a “high-quality” survey, in this discussion we consider the characteristics that might contribute to such a classification, taking into account current standards and practices in the field We propose that high-quality surveys are surveys that allow gathering data on the performance and safety of a device in a manner that allows comparison with established state-of-the-art benchmarks. This definition underscores the dependency of such surveys on state-of-the-art analysis. For instance, it is challenging to develop a high-quality survey that convincingly demonstrates a device’s performance and safety using PROs, if PROs are not recognized in the relevant medical field for the specific device application. This reliance on the state of the art extends to other aspects of survey methodology, such as sampling techniques, questionnaire design, data collection, data analysis, and interpretation. As explained in MDCG 2020-7 “justification other than ‘this should demonstrate the expected quality of evidence that we require,’ but without showing a statistical rationale, are not acceptable”. As the example of high-quality surveys makes clear, the boundaries between surveys and clinical investigations are not always clear-cut. Manufacturers often invest substantial resources in positioning their data collection efforts as surveys rather than clinical investigations, as surveys are perceived to offer a reduced regulatory burden. Yet, in many cases, the regulatory effort required to conduct a survey and meet regulatory obligations is comparable to that of clinical investigations. Consider prospective PMCF surveys. Even when the survey content is not focused on assessing a device’s performance and safety—and therefore does not qualify as a clinical investigation—conducting the survey still requires planning, recruiting, analysis, reporting, and obtaining participant consent for data collection and processing. The main difference between a PMCF survey and a PMCF observational study thus lies in the process of obtaining ethics committee approval. Since ethics committees are unlikely to deny approval for data collection involving a certified device used according to standard practices that ensure safety, the expected ease of conducting surveys compared to clinical investigations may be overstated. However, in an attempt to structure data collection outside the scope of clinical evaluations, manufacturers often drastically limit the scope of their survey questions, missing valuable opportunities to gather the data they truly need. This is especially detrimental for manufacturers of legacy devices who urgently require robust clinical data for MDR submissions. In the past, there was relatively limited scrutiny regarding the correct classification of surveys or usability studies within the medical device sector, likely as a means to avoid overburdening manufacturers with regulatory demands. However, this trend is changing as notified bodies are increasingly focusing on these areas, particularly following the release of MDCG 2021-6. As the medical device sector continues its journey toward best clinical practices, this level of scrutiny is only expected to increase.




