Introduction
MDR Article 2.12 defines “intended purpose” as: “the use for which a device is intended”. That statement is quite circular for a definition. While the remainder of Article 2.12 emphasizes the need of consistency between information material, clinical data and intended purpose, it still does not provide much help when it comes to building an intended purpose for CE-certification. However, there is no reason to despair. First, you probably have more experience with intended purposes than you think. The pharmaceutical industry has been drafting intended uses for decades. When you buy a medicine, you will find a pamphlet explaining what the medication is for, who should take it, when to take it, and so on. This is the intended use of the medication. While there are differences between the intended uses of medicinal products and those of medical devices, reviewing this information can give you an idea (and also inspiration) of what an intended purpose might look like. Furthermore, in this paper, we provide a clear methodology for defining the intended purpose of medical devices, along with examples and best practices. Before we dive into the methodology, let’s answer a few we are frequently asked.
2 The structure of the intended purpose
The MDR does not explicitely specify a structure of the intended purpose. However,
the MDCG points to the section of the MDR that they consider a specification of the content of intended purpose. Specifically, MDCG 2022-9 points to MDR Annex II, Part 1.1(c) as the requirements on the content of the intended purpose. This content includes “the intended patient population and medical conditions to be diagnosed, treated and/or monitored and other considerations such as patient selection criteria, indications, con- traindications, warnings”. We can find confirmation of the MDCG approach in another part of the MDR, specifically Annex I, Part 23.4(b). This MDR section specifies that the IFU should include“the device’s intended purpose with a clear specification of indications, contra-indications, the patient target group or groups, and of the intended users, as appropriate.” This structure is reminiscent of that specified in Annex II, Part 1.1(c). All-in-all, both MDR sections, highlight a similar structure, as follows:
1. Indication(s)
2. Target population(s)
3. Contraindications and warnings
4. Intended user(s)
In this document, we propose a slightly modified structure for the intended purpose with
two additional points:
1. Generic device group

2. Indication(s)
3. Target population(s)
4. Contraindications and warnings
5. Intended user(s)
6. Relevant safety and performance information
In the remaining of the document, we will analyze each element separately.
3 Generic device group
MDR Article 2.7 defines generic device group “a set of devices having the same or similar intended purposes or a commonality of technology allowing them to be classified in a generic manner not reflecting specific characteristics”. The regulation does not explicitly require the inclusion of the device group in the intended purpose. However, if the medical literature specifies standardized nomenclature to describe your device group, it is useful to provide this information at the start of the intended purpose. Consider an example. Devices that use UVA or UVB light to treat skin conditions are commonly referred to in the medical literature as “phototherapy devices”. Using this terminology ensures that users immediately understand the type of device being described. The intended purpose of such a device can thus begin with this information:
- Example : Phototherapy device
- = Generic device groupe
4 Indication(s)
We can categorize device indications into two types:
• Condition-based indications
• Procedure-based indications
The following sections provide a detailed analysis of each indication type.
4.1 Condition-based indications
MDR Article 2.1 clarifies the medical purposes—such as prevention of disease, treatment of a disability, etc.—covered by the regulation. These purposes are summarized in Table 1. A large number of medical devices achieve their medical purposes for a defined set of conditions. Here we use the term “condition”, which is not explicitly defined in regulatory guidance but is widely used in medicine to describe a broad category of health issues, including diseases, injuries, and disabilities, pathological processes, etc. For these devices, the indication can be specified using their medical purpose. Let’s consider some examples.
Phototherapy devices are used to treat dermatological disorders, such as psoriasis. For diseases, the permitted purposes include treatment, alleviation, diagnosis, prevention, monitoring, prediction, and prognosis. Phototherapy devices are typically used for treatment. The indication of a phototherapy device, therefore, is to treat psoriasis. Here a possible formulation of intended purpose for such a device:
- Example : Phototherapy device
- = Indication
Hearing aids are an example of device for compensation of a disability
- Example : Bone-conduction hearing aid
- = Indication
A limb prosthesis serves as a replacement for a missing or impaired part of the
anatomy.
- Example : Hand prosthesi
- = Indication
Devices that are medical device software or that include medical device software (see MDCG 2019-11 for a definition of medical device software) might achieve their intended purpose indirectly by providing information to the user. In such cases, it is important to emphasize this in the intended purpose. This is done by using the formulation from the first part of MDR Annex VIII, Part 6.3 (Rule 11): “provide information which is used to take decisions”. For example:
- Example : Lung-cancer detection AI system
- = Indication
A device can have several indications simultaneously. For example, the same device could treat a condition or help preventing it:
- Example : Continuous passive motion
- = Indication
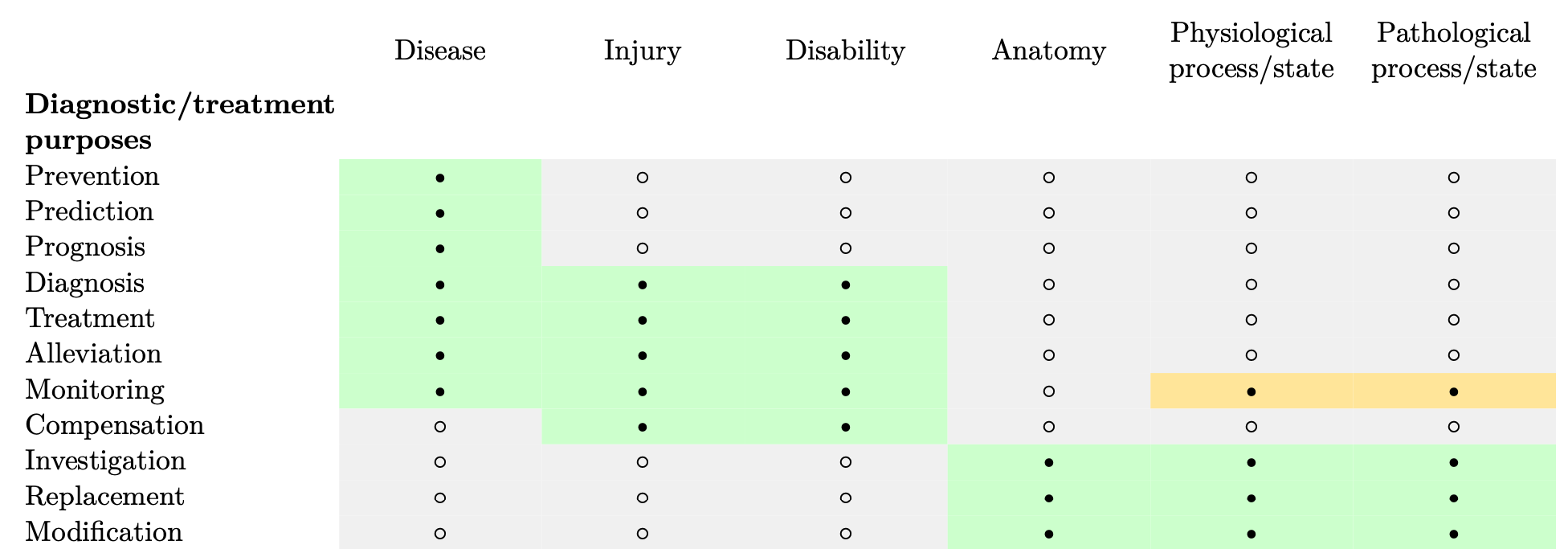
Table 1: Medical purposes defined in MDR Article 2.1, definition of “medical device” provided in (•= combination applicable, ◦ = combination not applicable). The combinations in orange are provided in MDR Annex VIII. Monitoring of physiological processes is not specified as a medical purpose in Article 2.1. However, MDR Annex VIII extends the are of application of “monitoring” to phsyiological conditions, state of health, illnesses or congenital deformities, physiological processes, vital physiological processes and vital parameters.
Continue reading
Download the full document for FREE!
Explore the complete article: How to build an Intended purpose for MDR for FREE!





